Products
- > 50.000€, external funding assisted by our team;
- Runtime > 1 year and divided by several work packages;
- Iterations in development;
- Assistance in technical functionality testing;
- Technological Readiness Level 3-7;
- Documentation for METC or CE marking;
- Production ready.
Matrix sublimator
M4I Mass spectrometry requested the design and fabrication of a robust prototype to sublimate matrix on to biologic tissue samples (TRL 6). This work involved 3D CAD design, fabrication, technical testing, IP documentation, technology transfer to company HTX.

Incubator stretcher
MUMC+ requested the design of an incubator stretcher for transporting neonates. This work involved 3D CAD design, fabrication, technical testing, TP and CE documentation achieving a TRL 7 level. Other hospitals have also purchased a system (e.g. LUMC).

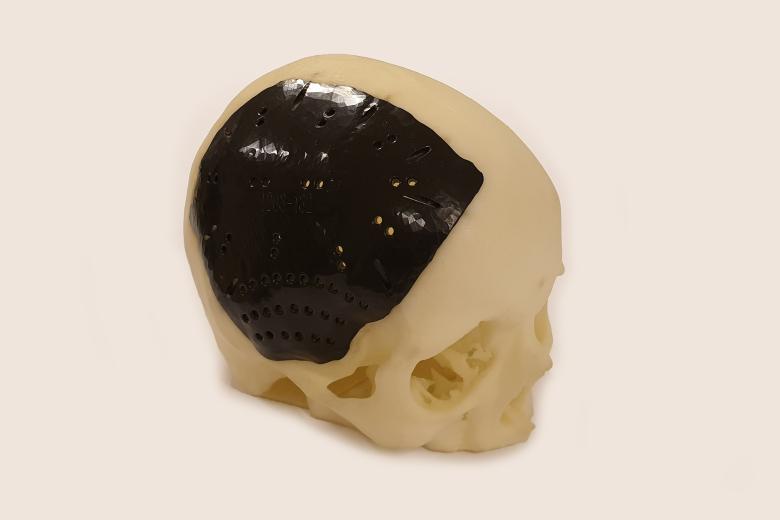
Skull implant medical device class III
Cranio-Maxillofacial Surgery, MUMC+ requested the design and fabrication of a patient specific skull implant, which has been placed in the patient. This work involved 3D CT data segmentation, 3D CAD design, fabrication and documentation according to ISO14358.
High resolution ECG recorder
Brightlands spin-off company Yourrhythmics requested assistance in redesign of their high resolution ECG recorder prototype for manufacturing and scale up at TRL 9.

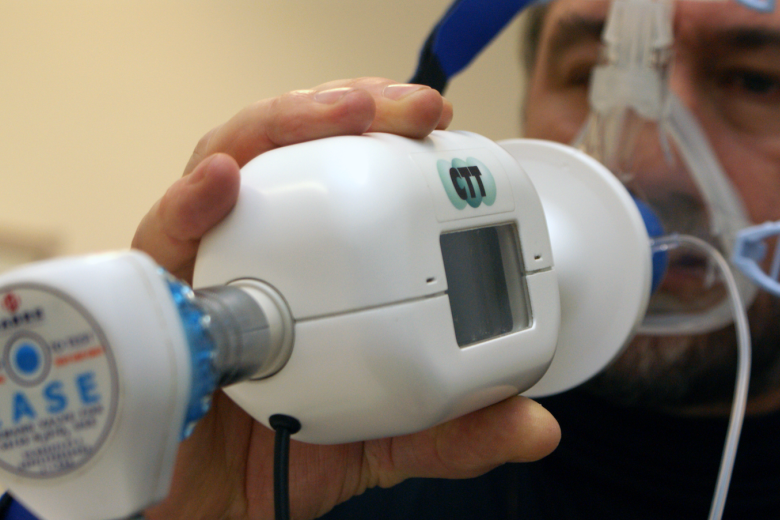
CO2 inhalation challenge model
For the Centre for Human Drug Research CHDR, a carbon dioxide tolerance tester was developed that delivers a controlled gas mixture through a protected inhalation system and is used to measure anxiolytic/panicolytic effects of new test compounds.
MOX data acquisition platform
Over the years, a stable data acquisition system was developed for accelerometry as well as for swift coupling with other sensors. This work involved electronic design, embedded software development, extensive testing and validation. TRL9.

Maastricht Instruments
Maastricht Instruments is a product incubator at the Brightlands Maastricht Health Campus. We offer research prototypes developed at Maastricht UMC+ to worldwide R&D institutes.
