Radical Medicine – Redefining Oxidative Stress / Pharmacology & Personalised Medicine
Reactive oxygen species (ROS) have been suggested as a major mechanism of diseases and a leading cause of death and disability worldwide. However, all major clinical trials, which tested the application of anti-oxidants in thousands of patients, have been disappointingly negative or even suggested serious side effects.
Here, we approach the oxidative stress hypothesis in completely different way: First, instead of letting ROS form and then scavenge them, we will identify their sources and prevent their formation in the first place. Second, we will define beneficial and essential signaling roles of ROS. In combination, this will result in unprecedented precision and molecular specificity. We will focus on cerebro-cardiovascular diseases (CCVD), such as myocardial infarction, coronary artery disease and stroke (the fastest growing and no 1 cause of death). Further, we envisage a substantial step forward in personalized medicine by offering for the first time predictive diagnostics (blood-based, in vitro and in vivo/imaging) and effective therapeutics. We also expect to be able to apply our findings and tools to several other areas of biology and medicine, in which ROS play physiological and pathological roles. Such common mechanisms are the missing link in the diseasome, which willlead to new disease definitions and an entirely novel approach to diagnostics, therapy and prevention.
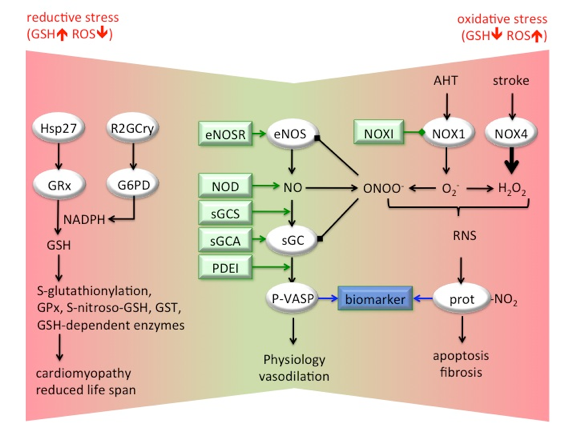
In CCVD, NADPH oxidase (NOX)-induced oxidative stress (increased superoxide, O2-, and hydrogen peroxide, H2O2, levels) affects NO-sGC signalling and vasodilatation in 3 ways: (i) scavenging of NO (with peroxynitrite, ONOO-, formation), (ii) uncoupling of eNOS, and (iii) oxidation/heme-loss of the NO-receptor, Fe(II)sGC. ROS and NO also form reactive nitrogen species (RNS), modifying cell components, including protein tyrosine nitration (prot-NO2), correlating with cellular apoptosis and fibrosis. These pathways can be assessed with biomarkers such as phospho-VASP (P-VASP) for physiologic NO signalling, and nitrotyrosin for RNS. Therapeutic options include inhibition of NOX (NOX1 in AHT, NOX4 in stroke), eNOS recoupling (eNOSR), sGC stimulation (sGCS), sGC activation (sGCA), and phosphodiesterase inhibition (PDEI). Anti-oxidants are no therapeutic alternative. They are ineffective or even harmful, possibly by causing reductive stress, i.e. unphysiological high glutathione (GSH) levels due to activation of glutathione reductase (GRx) by heat shock protein (Hsp)27 or glucose-6-phosphate dehydrogenase (G6PD)-derived NADPH. Increased GSH results in glutathione peroxidase (GPx)-dependent decreased ROS levels leading to S-(nitroso) glutathionylation and eventually to cardiomyopathy and reduced life span.
Participants: Pharmacology & Personalised Medicine
-

A.I. Casas Guijarro
Pharmacology and Personalised Medicine
