Vitrojet, indispensable for electron microscopes
Thanks to cryo-electron microscopy, scientists can see inside cells, all the way down to the molecular level. This revolution makes it possible to analyze the precise composition of the many thousands of proteins. It might also reveal the mysteries of how diseases such as Alzheimer’s or tuberculosis develop. Based in Maastricht and a spin-off of the Nanoscopy department of the FHML faculty at MUMC+, CryoSol-World is joining the revolution with a device that makes research material suitable for studying under a microscope, quickly and efficiently.
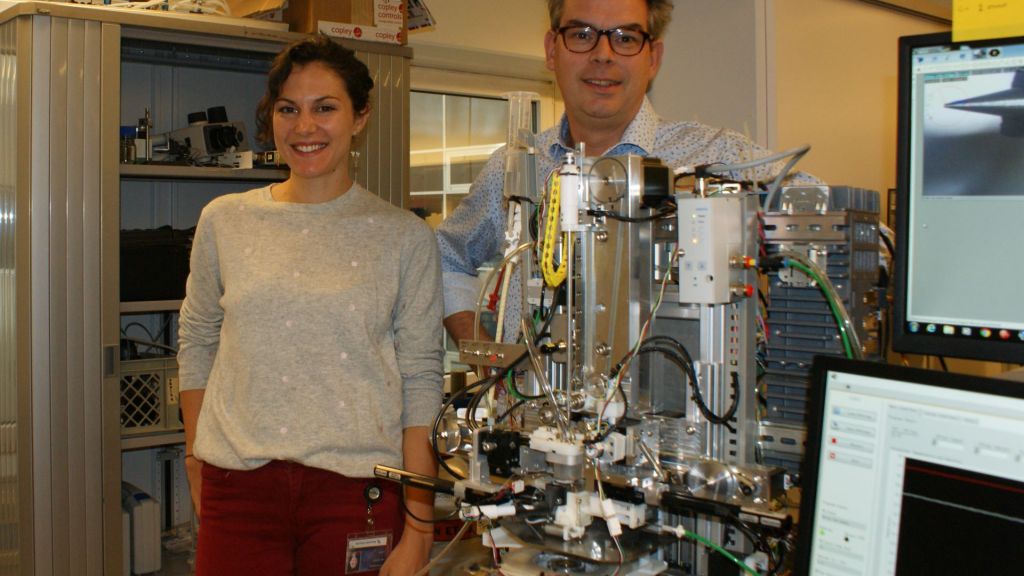
In 2001, Dr. Peter Frederik of FHML (Faculty of Health, Medicine and Life Sciences) worked with Maastricht Instruments, a spin-off of the technical division of IDEE of the medical faculty of Maastricht University, to develop the Vitrobot. This instrument freezes research material incredibly fast onto a small plate, making it suitable for studying under an electron microscope.
The Eindhoven-based manufacturer of electron microscopes FEI (now Thermo Fisher) quickly saw the potential of this development and purchased the license. There are now hundreds of Vitrobots in hospital laboratories and research centers all over the world. “An electron microscope allows you to see a lot more detail than you would with a standard light microscope,” explains Bas Lemmens of CryoSol-World, who was involved in the development of the Vitrobot together with Frank Nijpels. “Electron microscopes and cameras have undergone major improvements in recent years, and better software has been written to make 3D images as small as 0.2 nanometers visible. These developments have really accelerated the progress being made in the research method.”
Nobel prize
This is putting it mildly: last year, a team comprised of a Swiss, German and Brit won the Nobel prize for their research on using cryo-EM, as the method is known, to examine proteins at the molecular level. “Fantastic,” continues Bas Lemmens, bioprocess technician. “The structures of the proteins in a human cell are made visible in one go. Viruses and bacteria may be dissected down to the smallest detail. Now that scientists can see the structure of cells and how they behave, this can serve as the foundation for the development of medicines and vaccines.”
Samples
The method is essentially simple. Research material such as a protein in solution is mounted or fixed to a slide in the Vitrobot and then viewed through a microscope. This fixation occurs by freezing the material at a high rate of speed. “Something often goes wrong during the first phase. Only one out of four samples ultimately ends up being suitable for obtaining an image and using it to collect data. With the other samples, we don’t find out until it is under the microscope that something went wrong during the freezing process, for example that the ice is full of crystals which prevents the microscope from recording images. Sometimes, a lot of research material gets lost during the current freezing process. This may not seem like such a bad thing, but it’s often incredibly time-consuming and expensive to isolate a protein, for example. Suppose that you only have access to a couple of microliters and you ruin these in the Vitrobot; this is disappointing for the scientist. The current devices also need a relatively large quantity of material in order to produce one sample.”

Stable
In short, the ultramodern cryo-EM would benefit from a better and more stable supply of samples. This is an excellent reason for the Nanoscopy researchers to work with CryoSol to develop a new generation of sample preparation technology, this time with the help of Frank Nijpels, who was there at the inception of the successful Vitrobot. The process was supervised by Professor Peter Peters, head of the Nanoscopy department.
For a little less than three years, the team, supervised by Dr. Bart Beulen, worked on the new device, christened Vitrojet. The first prototype is now ready, and a company has been set up to finish developing the device and launching it on the market: CryoSol. “We expect to be able to place the first devices in mid-2019 with labs in Germany, the UK, China and the US, among other countries,” says Bas Lemmens, who works as a business developer at CryoSol.
Automated
It shouldn’t be hard to persuade people. “No, the Vitrojet is a fully automated system in which 90 percent of the samples are suitable for the microscope. This is a vast improvement over the 25 percent that are currently suitable. This is because our work is automated, and we use a different method for applying and freezing the samples. When you think that an electron microscope costs 6000 Euros per day to use, then we’re talking about a major time and cost savings on an annual basis. You could just purchase an additional microscope to meet your research objectives, but one of these microscopes costs around six million Euros. I can’t really say anything about the price right now, but the Vitrojet will be a much better solution. We believe that the Vitrojet can be just as successful as its predecessor.”
Also read
-
Gera Nagelhout is, in many respects, not a typical professor. She was the first in her family to attend university, and at the age of 34 was appointed endowed professor of Health and Wellbeing of People with a Lower Socioeconomic Position.
-
It’s a major step forward on the road to sustainable agriculture and healthy food.
-
The Netherlands Organisation for Scientific Research (NWO) is allocating more than 17 million euros in subsidies for the further development of a Dutch network for electron microscopy (NEMI). Almost 5 million of this will go to UM. From Maastricht, the M4I institute of university professors Ron...


